OPPOSITE
Summary of the Project
Gastric cancer is the third most common cause of cancer related death worldwide. Every year around 725 000 people die due to gastric cancer. Due to a lack of early symptoms and no effective screening programs most gastric and esophagogastric junction cancers are diagnosed in a locally advanced or metastatic stage in Western countries. Even in localized stages long term survival is below 50 %.
Perioperative systemic treatment has significantly changed the management of locally advanced tumors resulting in a survival benefit of about 15% in comparison to surgery alone. However a relevant part of patients does still not show a major response to perioperative treatment and is exposed to a potentially ineffective and toxic treatment.
This pilot trial examines the feasibility of a molecular classification from the initial biopsy. In parallel the predictive value of a patient-derived organoid model with respect to treatment efficacy is explored. In patients not responding to neoadjuvant treatment (showing no or only a minor histological response), further individual treatment recommendations based on molecular alterations will be given.
We believe that molecular stratification in combination with in vitro response testing in organoids has the potential to aid therapy stratifications and thereby improve the outcome of gastric cancer patients.
Scientific Goals
- Classification of tumors to molecularly defined subtypes and correlation with histological response to neoadjuvant systemic treatment
- Analysis of the predictive value of the gastric cancer organoid model to predict patient’s histological Response
- Understand mechanisms of resistance against perioperative systemic treatment
- Develop effective treatment options in non-responders
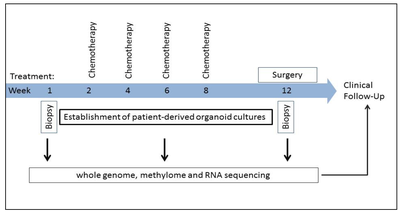
Study Design
Giessler K*, Kleinheinz K*, Huebschmann D, Balasubramanian G, Dubash TD, Dieter SM, Siegl C, Herbst F, Weber S, Hoffmann CM, Fronza R, Buchhalter I, Paramasivam N, Eils R, Schmidt M, von Kalle C, Schneider M, Ulrich A, Scholl C, Fröhling S, Weichert W, Brors B, Schlesner M, Ball CR#, Glimm H#. Genetic subclone architecture of tumor clone-initiating cells in colorectal cancer. J Exp Med. 2017;214:2073-2088.
Ball CR*, Oppel F*, Ehrenberg KR*, Dubash TD, Dieter SM, Hoffmann CM, Abel U, Herbst F, Koch M, Werner J, Bergmann F, Ishaque N, Schmidt M, von Kalle C, Scholl C, Fröhling S, Brors B, Weichert W, Weitz J, Glimm H. Succession of transiently active tumor-initiating cell clones in human pancreatic cancer xenografts. EMBO Mol Med. 2017;9:918-932
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Konigsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jager E, Monig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016; 17:1697-1708
Stange DE*, Koo BK*, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357-68.
Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665-9.
Contact
PD Dr. Georg Martin Haag
NCT, Dep. of Medical Oncology, University Hospital Heidelberg
Phone: +49 6221 56 37630
Email: GeorgMartin.Haag(at)med.uni-heidelberg.de
PD Dr. Dr. Daniel E. Stange
Dept. of Visceral, Thoracic and Vascular Surger, University Hospital Dresden
Phone: +49 351 458 18263
Email: Daniel.Stange(at)uniklinikum-dresden.de
Prof. Dr. Hanno Glimm
NCT, Dep. of Translational Oncology, DKFZ HD
Phone: +49 6221 56 6979
Email: hanno.glimm(at)nct-heidelberg.de