NCT MASTER-Program
Comprehensive analysis of every cancer patient at every major decision milestone
The MASTER (Molecularly Aided Stratification for Tumor ERadication) Program is a central platform for comprehensive, multidimensional characterization of young cancer patients and patients with rare cancers seen at NCT/UCC Dresden, NCT Heidelberg, or one of the sites of the German Cancer Consortium or the OneNCT.
The ultimate goal of this multidisciplinary effort is to enable clinically meaningful precision cancer medicine by applying current and future technologies that capture the molecular, cellular, and functional properties of individual human tumors in an integrated fashion. To this end, the NCT MASTER team of physicians, scientists, and study assistants as well as investigators at the Department of Translational Oncology, Institute of Pathology Heidelberg, the DKFZ Genomics and Proteomics Core Facility, the DKFZ Omics IT and Data Management Core Facility, the Division of Applied Bioinformatics at DKFZ, as well as the Department for Translational Medical Oncology, the Department of Human Genetics and the Pathology Department of the NCT in Dresden work jointly on strategies for rapid-turnaround molecular profiling and streamlined data acquisition and analysis in a clinical setting.
As of June 2025, more than 5000 younger patients with advanced-stage malignancies and patients with rare tumors have been enrolled and discussed in a molecular tumor board dedicated to the identification of novel treatment approaches based on molecular profiling. Evaluation of the data has allowed formulation of evidence-based recommendations for clinical management in around 80% of cases.
For more information on how to participate in NCT MASTER, visit "Vom Gen zur Therapie" .
As a research program and registry trial the MASTER Program always strives to push the boundary of multi-omics stratification. Exploring the capabilities of novel methods we aim to gather new insights through multi-faceted molecular diagnostics.
Proteomics: Phosphoproteomics is an emerging technology that enables the direct measurement of protein abundance and activity (via phosphorylation) in tumor samples. In collaboration with colleagues from the Kuester Lab at the Technical University of Munich, we integrate this additional layer of information with genomic, transcriptomic, and methylation data. To date, more than 2,200 samples have been analyzed. The data discussed in the molecular tumor board provide valuable insights into the functional relevance of genetic alterations and can reveal aberrant kinase activity or protein expression that remain undetected by other omics layers. The team at TU Munich has developed 49 Tumor Proteome Activity Status (TOPAS) scores, designed to reliably identify aberrant kinase activity across 20 receptor tyrosine kinases (RTKs) and 29 cytoplasmic or nuclear kinases in individual patient samples. These analyses aim to enhance patient stratification for targeted therapies, particularly kinase inhibitors, antibodies, and antibody drug conjugates (ADCs), that act on specific proteins and signaling pathways. https://www.mls.ls.tum.de/proteomics/home/
Functional Testing: Functional drug sensitivity testing enables the direct assessment of therapeutic vulnerabilities in patient tumor samples and complements molecular sequencing data within the MASTER program. The assay can be performed on viable tumor material from different sources, including primary tissue and patient-derived cultures. This high-throughput platform enables functional profiling across more than 85 FDA-approved and investigational compounds, focusing on clinically relevant and targeted agents, with the panel continuously expanding. Identified treatment sensitivities are jointly reviewed and discussed in the molecular tumor board to support individualized therapy decisions.
Immunoprofiling: The composition of the tumor immune microenvironment is crucial for understanding a tumor's response to cancer immunotherapy. Together with the Department of Pathology and the Immune Monitoring Unit at the NCT Dresden, we determine important immune cell subsets (like T cells, B cells, dendritic cells, and macrophages), their phenotype, and their spatial distribution. Using the multi-spectral technology of the PhenoImagerHT (Akoya) and cyclic staining via the MACSima imaging platform (miltenyi), we simultaneously measure multiple markers that provide information about immune cell activity and insights into the state of the tumor (“hot”/“cold”). This helps physicians to evaluate recommendations for immunomodulatory therapies such as checkpoint inhibitors. https://www.nct-dresden.de/en/research/core-units/nctucc-immune-monitoring-unit?set_language=en
Rapid implementation of novel technologies for biology-guided stratification of adult cancer patients.
“Precision oncology” describes the ability to predict which patients will likely respond to specific cancer therapies based on increasingly accurate, high-resolution molecular diagnostics as well as the functional understanding of individual tumors.
MASTER provides a complete workflow for selection and consenting of patients, sample processing, whole-exome/genome and RNA sequencing, bioinformatic analysis and genomics-guided clinical decision making by a molecular tumor board composed of members with expertise in clinical oncology, pathology, molecular genetics, bioinformatics, medical genetics and counseling, pharmaceutics, and bioethics. The Department of Human Genetics in Dresden systematically evaluates the impact of germline mutations affecting known cancer genes within the MASTER cohort.
Since most of the alterations identified in human cancers have unknown functional consequences and can therefore not directly be interpreted regarding their suitability as therapeutic targets, separating “driver” mutations from biologically neutral “passenger” alterations is critical for the translation of genetic information into the clinic. Furthermore, the therapeutic value of known oncogenic alterations may vary depending on tissue context. To address these challenges, we jointly investigate the functional role of genetic alterations predicted to be damaging in appropriate experimental systems in Heidelberg and Dresden, followed by analysis of the phenotypic consequences. The intermediate goal of these studies is to establish a versatile platform for rapid functional testing of mutations identified in individual cancers and to develop a continuously evolving, “learning” system to support treatment decisions at NCT.
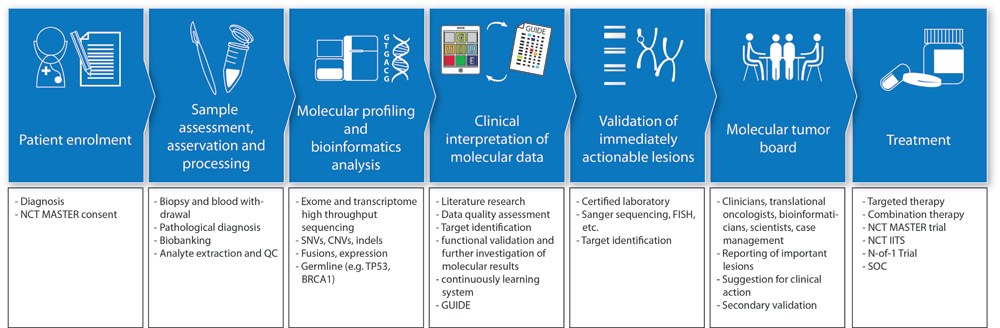
NCT Master Workflow © Daniela Richter
Swift translation of innovative high-throughput diagnostics into clinical practice An important strategic aim of the NCT in Dresden and Heidelberg is the development of novel concepts for the systematic translation of molecular information into clinical action. The NCT PMO (Precision Medicine in Oncology) Program provides a direct path from latest-generation molecular diagnostics within NCT MASTER to prospective, controlled clinical trials that will assess the efficacy of biology-guided cancer therapies and give patients access to targeted drugs that are otherwise not or only rarely available.
MASTER can also be the entry point for inclusion into other ongoing clinical trials across Germany and Europe. The molecular tumor board regularly provides information on available trial options that match the patients tumor profile.
>> Overview of available trials in Dresden