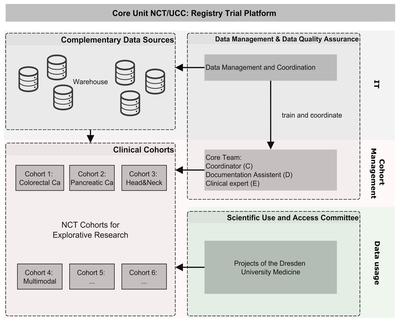
NCT/UCC Registry Trial Platform
Registry of clinical cancer cohorts for translational research at NCT/UCC Dresden and beyond
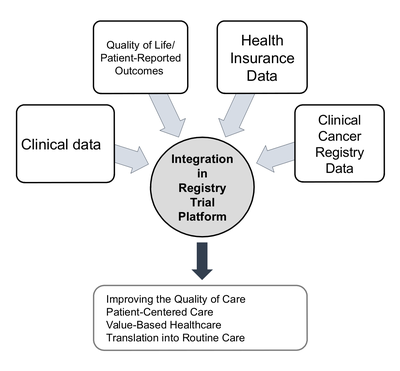
The aim of the platform is to establish a comprehensive disease-related retrospective and prospective data collection for each patient in the style of published ICHOM standard sets for oncological diseases. It includes clinical, patient-reported, healthcare insurance and registry data. Use of intelligent IT solutions and data warehouse technologies minimizes the effort required from the clinical staff. There is no need for additional elaborate clinical documentation. A core team was established to take responsibility for all processes, organizing and carrying out follow-up appointments and ensuring high data integrity.
We are assembling prospective clinical cancer cohorts to better understand the outcomes in routine care and promote back translation of study findings to accelerate medical innovation for patients.
We are starting with four cancer-specific cohorts (colorectal, pancreatic, head and neck and multimodal tumors) and making a comprehensive, high-quality dataset available for these oncological clinical settings as a prototype for further clinical cohorts at NCT/UCC Dresden and beyond.
Background
Cancer is the most common cause of death for middle-aged men and women in Germany and the second leading cause of death for children and adolescents. Innovative treatments are likely to modify cancer into a chronic rather than fatal disease in increasing numbers of patients. Therefore, ensuring the provision of high-quality, patient-centered care is a major challenge for the healthcare system. The analysis of prospective cohorts combines controlled, prospective observations with data on routine patient care as a method of translational research. Furthermore, clinical cohorts and registries serve as an excellent basis for embedding clinical studies. Back translation of the findings from healthcare research through prospective cohort studies into clinical research has the potential to advance medical innovation faster, more effectively and above all more purposefully. Therefore, it must be a central component of cutting-edge oncological research. The goal of the platform is to provide an optimal data basis for this kind of research.
Datzmann T, Schoffer O, Schmitt J, Böhme H, Fritzmann J, Distler M, Ubbelohde U, Giehl-Brown E, Henke T, Krause M, Glimm H, Bornhäuser M, Weitz J. Long-term observation of patients with cancer – an entity-independent registry for healthcare and translational research at the University Medicine Dresden (Cancer-Reg-VT). Gesundheitswesen 2023; 85 (Supp. 3): S226-S234.
Head
NCT/UCC Dresden
and
Center for Evidence-Based Healthcare, Medical Faculty Carl Gustav Carus, TU Dresden
Phone: +49 (0)351 458-6495
Email: jochen.schmitt(at)ukdd.de
PD Dr. rer. nat. Olaf Schoffer (Oncological Health Service Research)
Center for Evidence-Based Healthcare, Medical Faculty Carl Gustav Carus, TU Dresden
Phone: +49 (0)351 458-6494
Email: olaf.schoffer(at)ukdd.de
Coordination
NCT/UCC Dresden
Phone: +49 (0)351 458-17774
Email: daniela.piontek(at)nct-dresden.de
Staff
NCT/UCC Dresden
Phone: +49 (0)351 458-4127
Email: heiko.böhme(at)nct-dresden.de
N.N. (Medical Documentation)
NCT/UCC Dresden
Phone: +49 (0)351 458-3073
Email: rtp(at)ukdd.de