DELPHI
Summary of the Project
Standard treatment for locally advanced head and neck squamous cell carcinoma (HNSCC) is surgery followed by radio- or radiochemotherapy dependent on clinical risk factors. Patients with HPV16(+) HNSCC show higher tumor cure rates after primary radiochemotherapy than HPV(-) tumors. This applies also for postoperative radiochemotherapy with >95% cure rates 5 years after treatment.
While HPV(+) HNSCC patients are young, severe late toxicity is frequent after multimodal treatment with 21% and 42% at 3 and 5 years. As late toxicity correlate with radiation dose at organs at risk, a dose reduction in HPV(+) HNSCC appears promising to reduce toxicity and is justified in view of the high locoregional control rates.
The approach of a controlled stepwise reduction of radiation doses has been successfully applied in the past in low-risk Hodgkin lymphoma patients, where the lower radiation doses were finally demonstrated as superior to higher doses with similar cure but lower late toxicity rates. A similar concept is planned in the present trial with the present phase I trial being the first step.
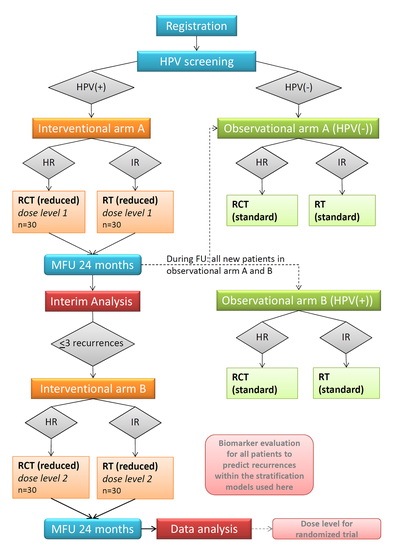
Figure 1: Flowchart of the trial
Abbreviations: HPV = Human papilloma virus, HR = high risk (clinical factors), LR = low risk (clinical factors), RCT = radiochemotherapy, RT = radiotherapy, MFU = minimal follow-up. *additional safety analyses in both risk groups and both dose levels after 10 and 20 patients
If the hypothesis can be verified, a randomized trial will be performed using the lowest effective radiation dose defined in this trial. This randomized trial would need to test the hypothesis that the lower radiation dose leads to lower late toxicity while efficacy stays constant.
Scientific Goals
- show safety of reduced-dose adjuvant radiochemotherapy
- show reduced late toxicity after reduced-dose adjuvant radiochemotherapy
**Linge A, Lohaus F, Löck S, …Abdollahi A, …Baretton GB, …Krause M, Baumann M (2016) HPV status, tumour volume, cancer stem cell marker expression and hypoxia status identify good prognosis subgroups in HNSCC after primary radiochemotherapy: results from a multicentre exploratory study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol 121, 364-71
***Linge A, Löck S, …Baretton G, …Baumann M, Krause M (2016) Independent validation of the prognostic value of CSC marker expression and hypoxia-induced gene expression for HNSCC after postoperative radiotherapy. Clin Transl Rad Onc 1, 19-26
**Linge A, Löck S, … Lohaus F, …Abdollahi A,… Baretton G, Baumann M, Krause M (2016) Low CSC marker expression and low hypoxia identify good prognosis subgroups in HPV(-) HNSCC after postoperative radiochemotherapy: a multicenter study of the DKTK-ROG. Clin Cancer Res 22, 2639-49
**Baumann M, Krause M, et al. (2016) Radiation oncology in the era of precision medicine. Nat Rev Cancer 16, 234-46
**Tinhofer I, …Abollahi A, Lohaus F, Krause M, …Baumann M (2016) Targeted next-generation sequencing of locally advanced squamous cell carcinomas of the head and neck reveals druggable targets for improving adjuvant chemoradiation. Eur J Cancer 57, 78-86
Dokic I., … Abdollahi A (2016) Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget 7, 56676-56689
**Tawk B, …Krause M, …Baumann M, …Abdollahi A (2015) Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol 118, 350-58
*Simon M, Habeck M, Büttner D, Habeck U, Nölling T, Krause M, Brix G, Willich N, Wenz F, Schmidberger H, Debus J, Baumann M (2015) Approval procedures for clinical trials in the field of radiation oncology [Genehmigungsverfahren klinischer Studien im Bereich der Radioonkologie]. Strahlenther Onkol 191, 909-20
**Balermpas P, …Krause M, Linge A, Lohaus F, … Abdollahi A, …Baumann M, …et al. (2016) CD81 tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicenter study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer 138, 171-181
**Lohaus F, Linge A …Abdollahi A, …Löck S, Krause M, Baumann M for the DKTK-ROG (2014) HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol 113, 317-23
Skripcak T, …Krause M, …Simon M, …Baumann M (2014) Creating a Data Exchange Strategy for Radiotherapy Research: towards federated databases and anonymised public datasets. Radiother Oncol 113, 303-09
Zips D, …Baumann M (2012). Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 105: 21-28.
Contact
Prof. Mechthild Krause
Director, Dept. of Radiation Oncology, University Hospital Carl Gustav Carus Dresden; Dept. of Radiooncology – OncoRay, Helmholtz-Zentrum Dresden-Rossendorf; OncoRay
Phone: +49 351 458 5441
Email: Mechthild.krause(at)uniklinikum-dresden.de
Dr. Dr. Amir Abdollahi
Dept. of Radiation Oncology, University Hospital Heidelberg, DKFZ
Phone: +49 6221 5639604
Email: a.amir(at)dkfz.de