Sponsors
Our Mission
We provide comprehensive service and an excellent infrastructure for conducting early phase clinical trials in patients with malignant diseases. We offer maximum safety to study-participants as well as professional assistance in conception, conduction and analysis of clinical trials to sponsors.
Affiliated to the National Center for Tumor Diseases (NCT/UCC) our dedicated ECTU is embedded in a network of specialized institutions with a long lasting record in all aspects of cancer related research:
- The University of Dresden – award winning in the German Excellence Initiative – offers the administrative scaffolding and access to a cluster of internationally renowned basic and translational research groups focusing on preclinical aspects of cancer research.
- The University Hospital Dresden, one of Germanys leading tertiary-care hospitals, top-scoring in the 2012 national Focus® Healthcare Ranking and comprising over 1300 hospital beds.
- The NCT/UCC itself, a DIN-ISO9001-2008 certified interdisciplinary center for the optimal treatment of patients with all kinds of malignant diseases, which offers regular tumor boards as well as in- and outpatient treatment facilities.
- The Coordination Center for Clinical Trials (KKS) Dresden, a core facility funded by the Medical Faculty, dedicated to support clinical trials and ensure trial conduct according to highest quality standards complying with national and international laws. The KKS is enabled to take over responsibility in the field of biometrics, clinical monitoring, database- and (e)CRF-management as well as pharmacovigilance.
- Alternatively, a diverse set of cooperating CROs may assist in study planning, conduction and analysis.
The NCT/UCC-ECTU has access to:
- Nationally and internationally recognized experts covering the whole spectrum of solid and hematologic cancers and attracting patients from all over Germany and neighboring countries.
- A team of committed clinical investigators, study nurses as well as administrative staff experienced in the conduction of clinical trials of all phases.
- Phase I experienced principal investigators covering solid as well as hematologic cancers.
- An accredited (certified according to 93/42/EWG and DIN EN ISO 15189) central laboratory offering comprehensive laboratory service 24 hours / 7 days a week.
- A DIN EN ISO 9001:2008 certified pathology department experienced in tumor histology serving as central pathology unit in substantial number of clinical trials.
- A GMP pharmacy licensed according to German Drug Law (AMG) for production, (re‑)packaging, blinding, randomization, export and distribution of investigational products.
- A JACIE-certified GMP laboratory licensed for the manufacturing, distribution and storage of cellular therapeutics (including investigational products) according to German Drug Law (AMG).
- A central database of all patients treated for malignant diseases at the NCT/UCC and cooperating private practice oncologists.
- A DIN EN ISO 9001:2008 certified tumor- and normal-tissue bank.
The NCT/UCC-ECTU is an independent department dedicated for early phase clinical trials. It offers 2 rooms for in-patient treatment equipped with 1 to 2 intensive care beds, comprehensive intensive care monitoring systems, private bathrooms, newspaper-, WLAN- and cable-TV-service. For maximum safety these rooms are located within a fully equipped intensive care unit offering 24 hour / 7 day on-site physician and specialist nursing service. Access to the ECTU is electronically controlled and restricted to authorized personnel. All medical staff is regularly trained in basic and advanced life support.
A team of GCP-trained, trial experienced physicians and specialized study nurses guarantee study conduct in accordance with national and international law as well as sponsor preferences. Synchronized clocks are installed in all rooms ensuring accurate timing of dosing and pharmacokinetic sampling. Special rooms are dedicated for the safe storage of investigational products and biologic specimen (at room temperature as well as at 4°C, -20°C and -80°C). All fridges and freezers are connected to a central surveillance system. For the work of monitors and auditors we offer dedicated rooms equipped with computers, printers and wireless internet connection.
The NCT/UCC-ECTU has privileged access to the treatment facilities of the outpatient departments for oncology as well as hematology which are located only a few meters away. These facilities are also equipped with cardiovascular monitoring systems and covered by the hospitals’ emergency service. A 24 hour / 7 day emergency hotline guarantees rapid assistance for ambulatory patients in need.
The NCT/UCC-ECTU and its cooperation partners provide comprehensive service from early planning to successful analysis, presentation and publication of each individual clinical trial. In a modular system we are happy to either assist your Clincial Research Organization / designated department or provide full CRO services in cooperation with our partners (incl. statistics support, database management, ethics committee and local authority communication). A fixed contact person accompanies all phases of a research project and coordinates the necessary activities.
We understand that timely subject recruitment is essential to the successful development of investigational products. As a tertiary care university hospital we treat a large number of cancer patients which are all registered in a central database. Moreover, regular tumor-boards and -conferences with an ever-growing network of private practice oncologists provide an additional access point for ambulatory patients. Taking these resources into account we conduct an ad-hoc feasibility analysis for every potential trial project and provide realistic commitments on recruitment numbers and timelines.
An electronic data management system is used for documentation in all patients treated at the university hospital. This ensures a seamless transition from in- to out-patient treatment as well as convenient source data access for monitors and auditors.
Dresden is located in the South-Eastern part of Germany approximately 200 km South of Berlin. It has an international airport servicing all major German hubs as well as several European capitals. On the ground it is well integrated into the German high-speed train- and motorway-network.
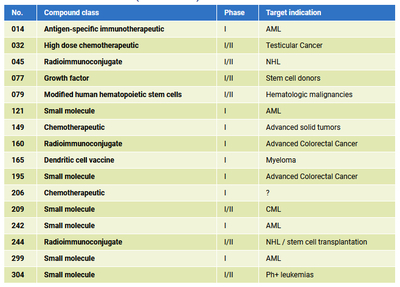
Timely contract procession is guaranteed at ECTU Dresden. Please send us your English, German or Bilingual contract drafts for medical and legal review.
- Short contract processing times
- Direct fixed contact person throughout the whole study
- Close monitoring and coordination of contract processing with all partners involved
- In case of parenteral study drugs we need a separate contract for our pharmacy
We focus on a straightforward, flexible and timely contract execution.