From the medical prescription of cancer drugs to their administration to the patient, there are many steps in the daily routine of a hospital that are carried out by numerous people, including doctors, nurses and pharmacists. Working in different systems and media increases the risk of potential errors. That's why the University Hospital Carl Gustav Carus Dresden was the first hospital in Germany to completely digitalize and standardize the medication process for oncological therapies. Patients at the National Center for Tumor Diseases Dresden (NCT/UCC) at Dresden University Hospital will in future benefit from the highest possible level of security in the prescription, production and administration of their chemotherapies and other medications.
The National Center for Tumor Diseases Dresden (NCT/UCC) is a joint institution of the German Cancer Research Center (DKFZ), the University Hospital Carl Gustav Carus Dresden, Carl Gustav Carus Faculty of Medicine at TU Dresden and the Helmholtz-Zentrum Dresden-Rossendorf (HZDR).
The clinical pharmacy of the University Hospital Carl Gustav Carus Dresden produces around 45,000 chemotherapy preparations per year - individually for each patient. These are often colorless liquids prepared in infusion bags. The potential mix-up of medications is one of numerous possible errors that can occur in the day-to-day supply of medications in hospitals. To minimize the risk of medication errors, Dresden University Hospital has now completely digitized and standardized the entire process from medication prescription to administration to the patient for the particularly sensitive area of cancer therapies. "Comprehensive digitization is an extreme leap forward in quality and increases safety for our patients," says Prof. Martin Bornhäuser, Director of Medical Clinic I at Dresden University Hospital and one of the Managing Directors at the National Center for Tumor Diseases Dresden (NCT/UCC).
Therapy requirements and laboratory findings, some of which were previously noted by hand or transmitted by fax, are now recorded uniformly in a software program. Doctors, pharmacists and nurses work together in one system. The Dresden University Hospital uses the "BD CATO TM" software for this purpose. "What is special is how extensively the software has been implemented at Dresden University Hospital. In the sense of a so-called closed loop medication management, it maps the entire oncological medication process," explains Prof. Gunnar Folprecht, Head of the Oncology Department of Medical Clinic I at Dresden University Hospital.
The numerous information and work steps that are recorded electronically include, for example, the selection of the treatment protocol suitable for the respective patient by the attending physician. The patient's laboratory values are transferred to the software via an interface to the hospital's laboratory program, which then calculates the dosage of medication. If predefined threshold values for the laboratory values - such as kidney values or the number of white blood cells - are exceeded, this triggers a warning message. After approval by the attending physician, the prescription is checked again for plausibility by a pharmacist and then each step in the production of the cancer medication in the pharmacy is documented within the system. The finished drug is labeled with an individual QR code. Only after approval by another pharmacist is the medication delivered to the oncology day clinic or the respective ward for administration.
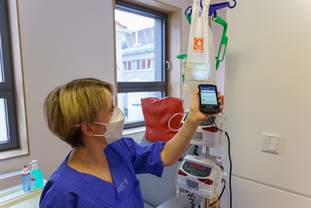
The administration of medications is also seamlessly documented: Using a special app ("BD Cato ReadyMed"), caregivers can call up the therapy plan of the respective patient on a mobile end device with additional hand scanner function. The plan shows in which order and at what time which medication must be administered. If a specific drug is to be administered, the nurse scans the barcode on the corresponding cancer drug. A patient-specific barcode is then scanned. This is generated individually for each patient and printed on the patient wristband. Such wristbands have been in use for inpatients for some time, while they are currently being introduced in the day clinic. The system automatically checks whether the medication and the patient match, whether the order of administration is correct, or whether the expiration date of the medication has passed. Only if everything is in order does the system give the green light for the drug to be administered. An individual barcode can also be printed out and scanned in each case for medications that were not manufactured via the clinic pharmacy and were barcoded there.
The comprehensive digitization of the entire medication process was established over a period of around three years. "As representatives of all the professional groups involved, we closely coordinated the exact design of the digital recording on a weekly basis. This close exchange has also once again significantly strengthened the cooperation between physicians, nurses and pharmacists," says Prof. Folprecht, who headed the responsible interdisciplinary project group. At the same time, all oncological therapy protocols, some of which previously differed from ward to ward, were standardized. The therapy protocols are precisely defined treatment concepts for specific clinical pictures, which specify, for example, the drugs to be administered, dosages, and the sequence and intervals of administration. "More than 600 therapy protocols for clinical routine and another 300 protocols for studies were standardized in close coordination between physicians and ward pharmacists. A truly mammoth task," says Dr. Holger Knoth, head of the hospital pharmacy at Dresden University Hospital. "We see ourselves as Dresden University Medical Center and as NCT/UCC as pioneers for safe drug supply and are pleased that the implementation process, which took several years, has now been successfully completed for the benefit of our patients," emphasizes Prof. Michael Albrecht, Medical Director of Dresden University Hospital.
Press contact:
Dr. Anna Kraft
Nationales Centrum für Tumorerkrankungen Dresden (NCT/UCC)
Presse- und Öffentlichkeitsarbeit
Tel.: +49 (0)351 458-5548
E-mail: anna.kraft@nct-dresden.de
www.nct-dresden.de